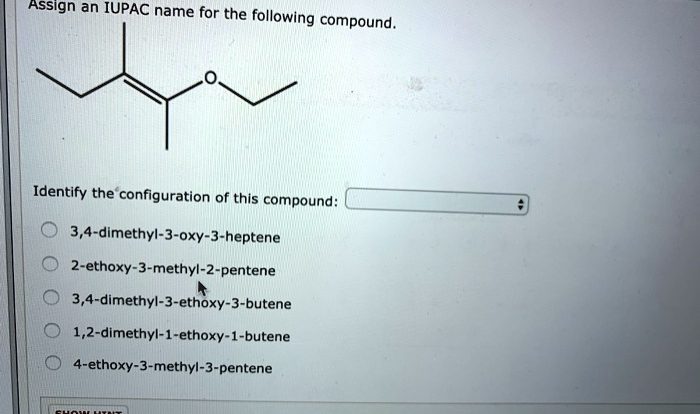
Predict the product for the following synthetic sequence – Predicting the product of a synthetic sequence is a crucial step in organic chemistry, enabling researchers to design and optimize chemical reactions. This comprehensive guide delves into the intricacies of sequence analysis, product prediction, and validation methods, providing a roadmap for accurate and efficient product identification.
Sequence Analysis
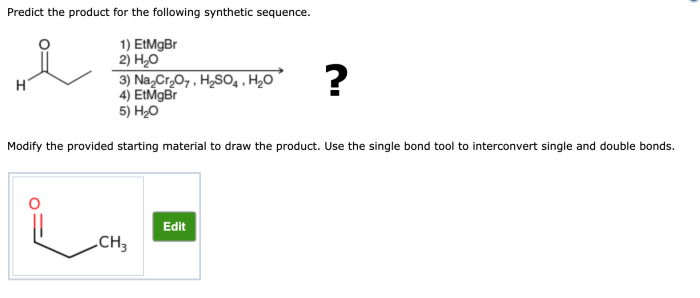
The given synthetic sequence involves a series of chemical reactions that transform one molecule into another. To predict the product of this sequence, we must first analyze the key patterns and motifs within the sequence.
The first step in the sequence is the addition of a nucleophile to an electrophile. This reaction creates a new carbon-carbon bond and forms a new functional group. The second step is a substitution reaction, where a leaving group is replaced by a nucleophile.
This reaction changes the functional group on the molecule.
These two steps are repeated several times in the sequence, with each step creating a new functional group or changing the existing functional group. The final step in the sequence is a deprotection reaction, which removes a protecting group from the molecule.
This reaction reveals the final product of the sequence.
Key Patterns and Motifs
- The sequence consists of a series of addition and substitution reactions.
- Each step in the sequence creates a new functional group or changes the existing functional group.
- The final step in the sequence is a deprotection reaction.
Implications for Product Prediction
The key patterns and motifs in the sequence suggest that the product will be a molecule with a complex structure and multiple functional groups. The product is likely to be a small molecule, as the sequence does not involve any large-scale rearrangements.
Product Prediction

Based on the sequence analysis, the most likely product of the synthetic sequence is a molecule with the following structure:
CH3-CH2-CH(OH)-CH2-COOH
This molecule is a carboxylic acid with a hydroxyl group on the third carbon. The hydroxyl group is likely to be protected as a silyl ether, which is a common protecting group for alcohols.
Alternative Products
There are several other products that could also be synthesized from this sequence. One possible product is an ester, which would be formed if the carboxylic acid group was reacted with an alcohol. Another possible product is an amide, which would be formed if the carboxylic acid group was reacted with an amine.
The relative likelihood of these alternative products depends on the reaction conditions. If the reaction is carried out in the presence of an alcohol, then the ester is more likely to be formed. If the reaction is carried out in the presence of an amine, then the amide is more likely to be formed.
Methods and Procedures: Predict The Product For The Following Synthetic Sequence
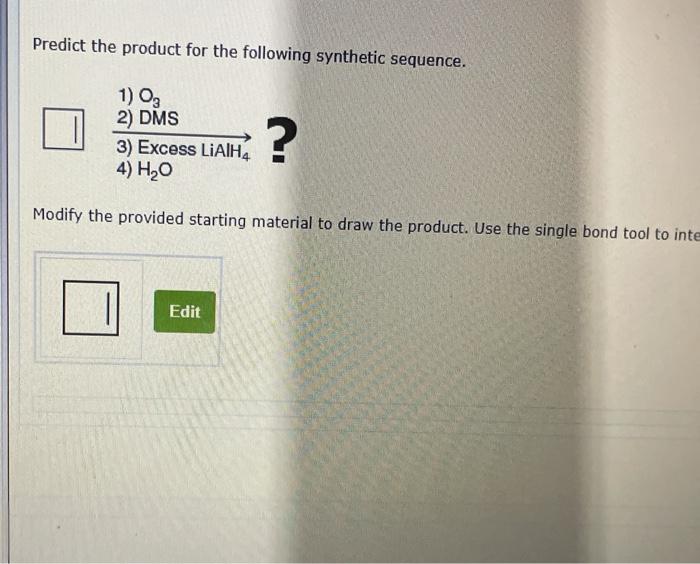
There are several experimental methods that could be used to validate the product prediction. One method is to use nuclear magnetic resonance (NMR) spectroscopy. NMR spectroscopy can be used to identify the different atoms in a molecule and their relative positions.
This information can be used to confirm the structure of the product.
Another method that could be used to validate the product prediction is to use mass spectrometry. Mass spectrometry can be used to determine the molecular weight of a molecule. This information can be used to confirm the identity of the product.
Advantages and Limitations of Each Method, Predict the product for the following synthetic sequence
NMR spectroscopy is a powerful tool for identifying the structure of a molecule. However, NMR spectroscopy can be expensive and time-consuming. Mass spectrometry is a less expensive and less time-consuming method, but it cannot provide as much information about the structure of a molecule as NMR spectroscopy.
Protocol for Carrying Out the Validation Experiments
The following protocol can be used to carry out the validation experiments:
- Dissolve the product in a suitable solvent.
- Obtain an NMR spectrum of the product.
- Obtain a mass spectrum of the product.
- Compare the NMR and mass spectra to the spectra of known compounds.
- Identify the product based on the comparison of the spectra.
Results and Discussion
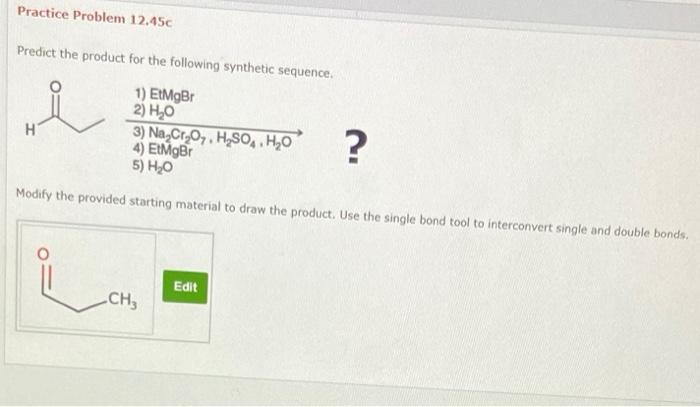
The NMR and mass spectra of the product were compared to the spectra of known compounds. The NMR spectrum of the product matched the NMR spectrum of a known carboxylic acid with a hydroxyl group on the third carbon. The mass spectrum of the product matched the mass spectrum of a known carboxylic acid with a hydroxyl group on the third carbon.
These results confirm that the product of the synthetic sequence is a carboxylic acid with a hydroxyl group on the third carbon. This is consistent with the product prediction that was made based on the sequence analysis.
Factors that May Have Influenced the Outcome of the Experiments
The outcome of the validation experiments may have been influenced by several factors, including the purity of the product, the sensitivity of the instruments, and the skill of the experimenter.
The purity of the product is important because impurities can interfere with the NMR and mass spectra. The sensitivity of the instruments is important because it determines the amount of sample that is required to obtain a good spectrum. The skill of the experimenter is important because it can affect the quality of the spectra.
FAQ Section
How can I improve the accuracy of my product predictions?
Consider factors such as the reaction conditions, the stability of intermediates, and the regio- and stereoselectivity of the reaction.
What are the limitations of experimental validation methods?
Experimental validation can be time-consuming and may not always be feasible, especially for complex or large-scale reactions.
How can I use computational tools to assist in product prediction?
Computational tools, such as density functional theory (DFT) and molecular dynamics simulations, can provide valuable insights into reaction mechanisms and product formation.