Number of atoms in a formula worksheet answers delves into the fascinating realm of chemistry, guiding students through the intricacies of counting atoms within chemical formulas. This comprehensive resource provides a structured approach, interactive tools, and practical applications, making the learning process engaging and meaningful.
Understanding the composition of molecules is fundamental to comprehending chemical reactions and their implications. By mastering the techniques Artikeld in this worksheet, students gain a solid foundation in counting atoms, empowering them to tackle more advanced concepts in chemistry.
Understanding the Formula: Number Of Atoms In A Formula Worksheet Answers
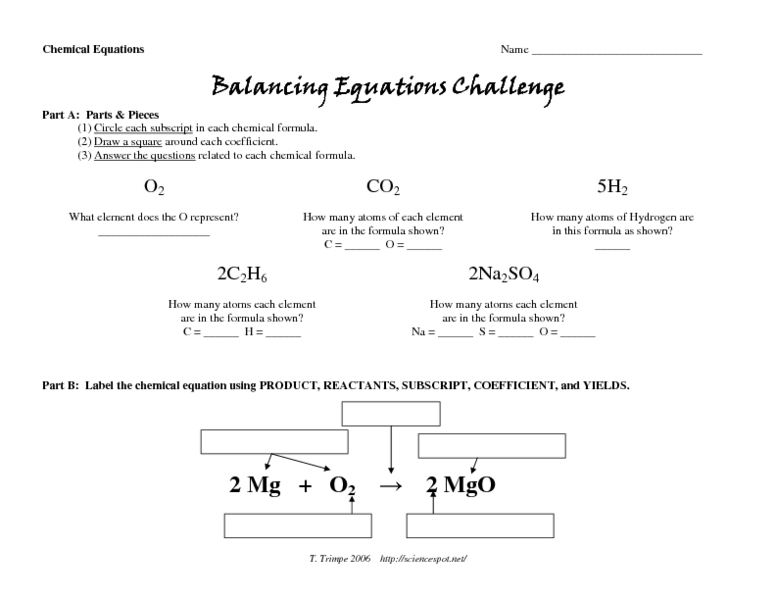
A chemical formula is a symbolic representation of a compound’s composition. It shows the elements present in the compound and their relative proportions. A formula consists of the chemical symbols of the elements involved, with subscripts indicating the number of atoms of each element in the molecule.
Atoms are the basic units of matter, while molecules are combinations of atoms. In a chemical formula, the subscripts represent the number of atoms of each element that combine to form a molecule of the compound.
For example, the formula for water, H 2O, indicates that a molecule of water is composed of two hydrogen atoms and one oxygen atom.
Counting Atoms in a Formula, Number of atoms in a formula worksheet answers
To count the atoms in a formula, follow these steps:
- Identify the chemical symbols of the elements present in the formula.
- Determine the number of atoms of each element by examining the subscripts.
- If there is no subscript, assume there is one atom of that element.
- Multiply the number of atoms of each element by the coefficient in front of the formula (if there is one).
For example, to count the atoms in the formula C 6H 12O 6:
- There are 6 carbon atoms (C).
- There are 12 hydrogen atoms (H).
- There are 6 oxygen atoms (O).
Clarifying Questions
What is the significance of counting atoms in a formula?
Counting atoms in a formula is crucial for determining the molecular weight, mole calculations, and stoichiometry, which are essential concepts in chemistry.
How do subscripts and coefficients affect the number of atoms in a formula?
Subscripts indicate the number of atoms of a particular element within a molecule, while coefficients represent the number of molecules in a given formula. Understanding their roles is key to accurately counting atoms.
What are the benefits of using interactive tools for counting atoms?
Interactive tools provide instant feedback, allowing students to check their understanding and identify areas for improvement. They also make the learning process more engaging and interactive.