Electron configuration answer key pogil delves into the fundamental principles governing the arrangement of electrons within atoms, providing a comprehensive understanding of their chemical properties and behavior. This guide explores the Aufbau principle, periodic trends, and exceptions to the Aufbau principle, equipping readers with a solid foundation in electron configuration.
Electron configurations play a pivotal role in determining the chemical properties of elements, including their electronegativity and ionization energy. By understanding electron configurations, chemists can predict the reactivity and bonding behavior of various elements, enabling the design of new materials and the development of innovative technologies.
Electron Configuration
Electron configuration refers to the distribution of electrons in the atomic orbitals of an atom. It describes the number of electrons in each energy level and subshell.
The Aufbau principle states that electrons fill the lowest energy orbitals first. As more electrons are added, they occupy orbitals with higher energy levels and subshells. The periodic trends in electron configuration show that elements with similar atomic numbers have similar electron configurations.
Electron Configuration of Elements
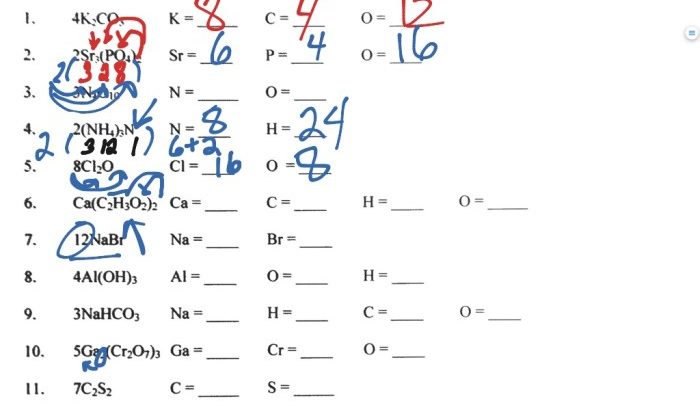
The electron configurations of elements in the first 20 rows of the periodic table can be determined using the Aufbau principle. The electron configuration of an element is written as a string of symbols representing the orbitals occupied by electrons, followed by the number of electrons in each orbital.
For example, the electron configuration of helium is 1s 2, indicating that it has two electrons in the 1s orbital.
Exceptions to the Aufbau Principle
There are some exceptions to the Aufbau principle. For example, the electron configuration of chromium is [Ar] 3d 54s 1, rather than [Ar] 3d 44s 2. This is because the half-filled 3d orbital is more stable than the fully filled 4s orbital.
Electron Configuration and Chemical Properties
Electron configuration affects the chemical properties of elements. Elements with similar electron configurations tend to have similar chemical properties. For example, the alkali metals (Group 1) all have one electron in their outermost shell, and they are all highly reactive.
The noble gases (Group 18) all have a full outermost shell, and they are all unreactive.
Electron Configuration and Spectroscopy
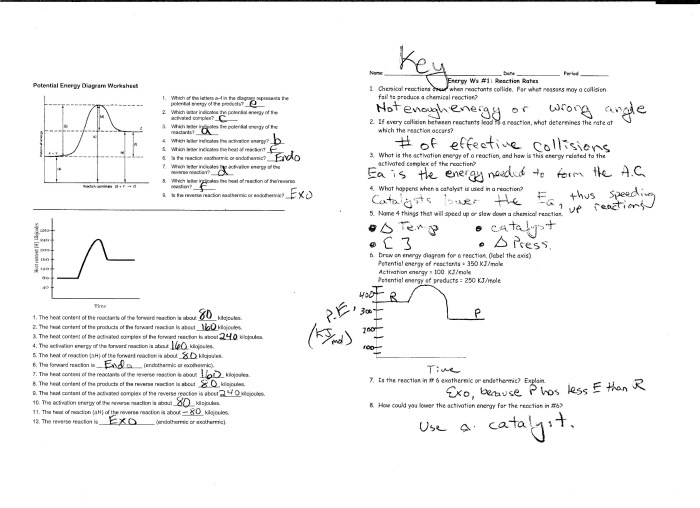
Electron configuration can be used to predict the spectra of atoms. The energy difference between two energy levels corresponds to the frequency of light that can be absorbed or emitted by an electron transitioning between those levels. The spectrum of an atom can therefore be used to determine its electron configuration.
Applications of Electron Configuration, Electron configuration answer key pogil
Electron configuration is used in a variety of fields, including chemistry, materials science, and astrophysics. In chemistry, electron configuration is used to predict the chemical properties of elements and to design new materials. In materials science, electron configuration is used to understand the properties of semiconductors and other materials.
In astrophysics, electron configuration is used to understand the spectra of stars and other celestial objects.
Answers to Common Questions: Electron Configuration Answer Key Pogil
What is electron configuration?
Electron configuration refers to the distribution of electrons within the energy levels of an atom, providing a detailed description of the arrangement and energy of electrons.
How does the Aufbau principle determine electron configuration?
The Aufbau principle states that electrons fill atomic orbitals in order of increasing energy, starting with the lowest energy level and progressing to higher energy levels.
What are the periodic trends in electron configuration?
Periodic trends in electron configuration include the increase in the number of electrons and energy levels across periods and the repetition of electron configurations in groups, leading to predictable chemical properties.